Thermostable enzymes for producing fuels and chemicals in thermophilic organisms
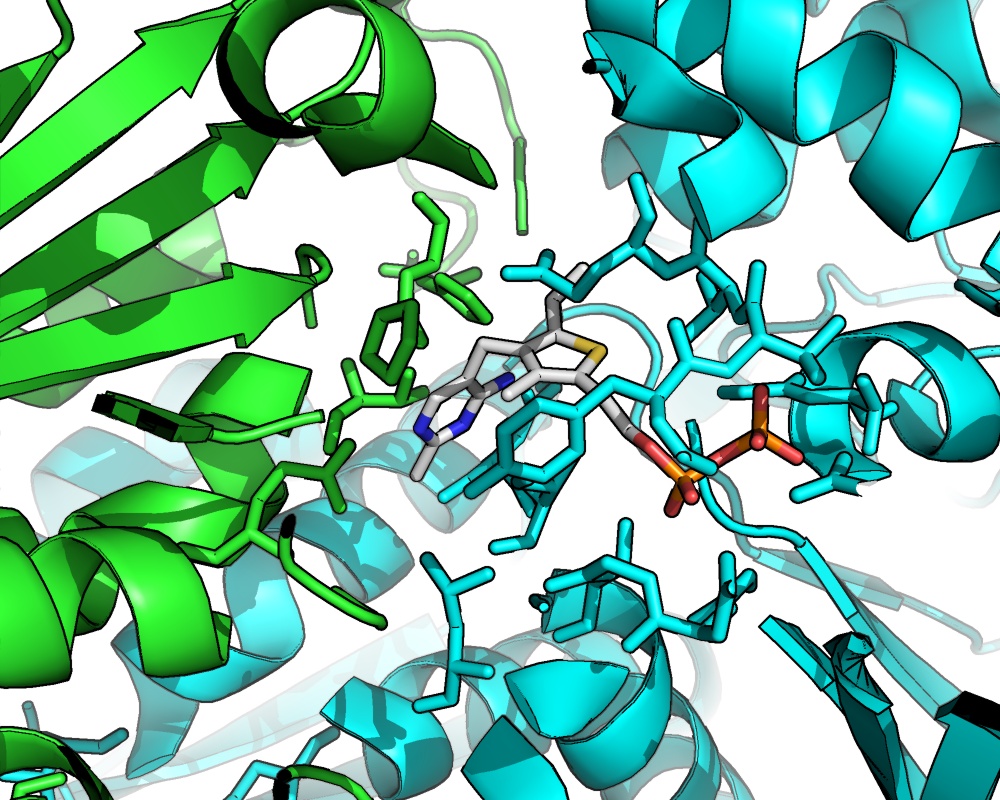 Thermophilic organisms have many potential advantages as hosts for producing fuels and chemicals: they can integrate well with product separation schemes, they are not susceptible to contamination by common mesophilic organisms, and there is the potential for higher catalytic activities and therefore production rates. We are currently investigating thermophilic pathways to produce isobutanol. We are exploring the genomes of different thermophilic bacterial strains for thermostable pathway enzymes. We are also using directed protein evolution to thermostabilize less-stable enzymes and optimize their activities in thermophilic hosts.
Thermophilic organisms have many potential advantages as hosts for producing fuels and chemicals: they can integrate well with product separation schemes, they are not susceptible to contamination by common mesophilic organisms, and there is the potential for higher catalytic activities and therefore production rates. We are currently investigating thermophilic pathways to produce isobutanol. We are exploring the genomes of different thermophilic bacterial strains for thermostable pathway enzymes. We are also using directed protein evolution to thermostabilize less-stable enzymes and optimize their activities in thermophilic hosts.
A key step in the isobutanol pathway is the decarboxylation of ketoisovalerate to isobutyraldehyde, a reaction which can be catalyzed by thiamine pyrophosphate-containing ketoacid decarboxylases. These enzymes are one focus of our engineering efforts. An interesting feature of these homodimeric proteins is the active site around the cofactor, which is comprised of amino acids from both subunits. Increasing the thermostability of this enzyme will enable its in vivo use at elevated temperatures. We are also establishing genetic tools for protein expression in thermophiles.
