Directed evolution of cytochrome P450 BM3
Cytochrome P450 BM3 (CYP102A1) from Bacillus megaterium is the parent enzyme for much of our work. This P450 has a number of features that make it ideal for directed evolution and synthetic applications. Unlike most P450s, BM3 is a soluble protein, it is easily produced in E. coli, and it is naturally fused to its reductase domain, whose inputs of electrons drive the P450 catalytic cycle. Additionally, it is perhaps the fastest P450 catalyst known, with a turnover number for arachidonic acid of 17,000 per minute.
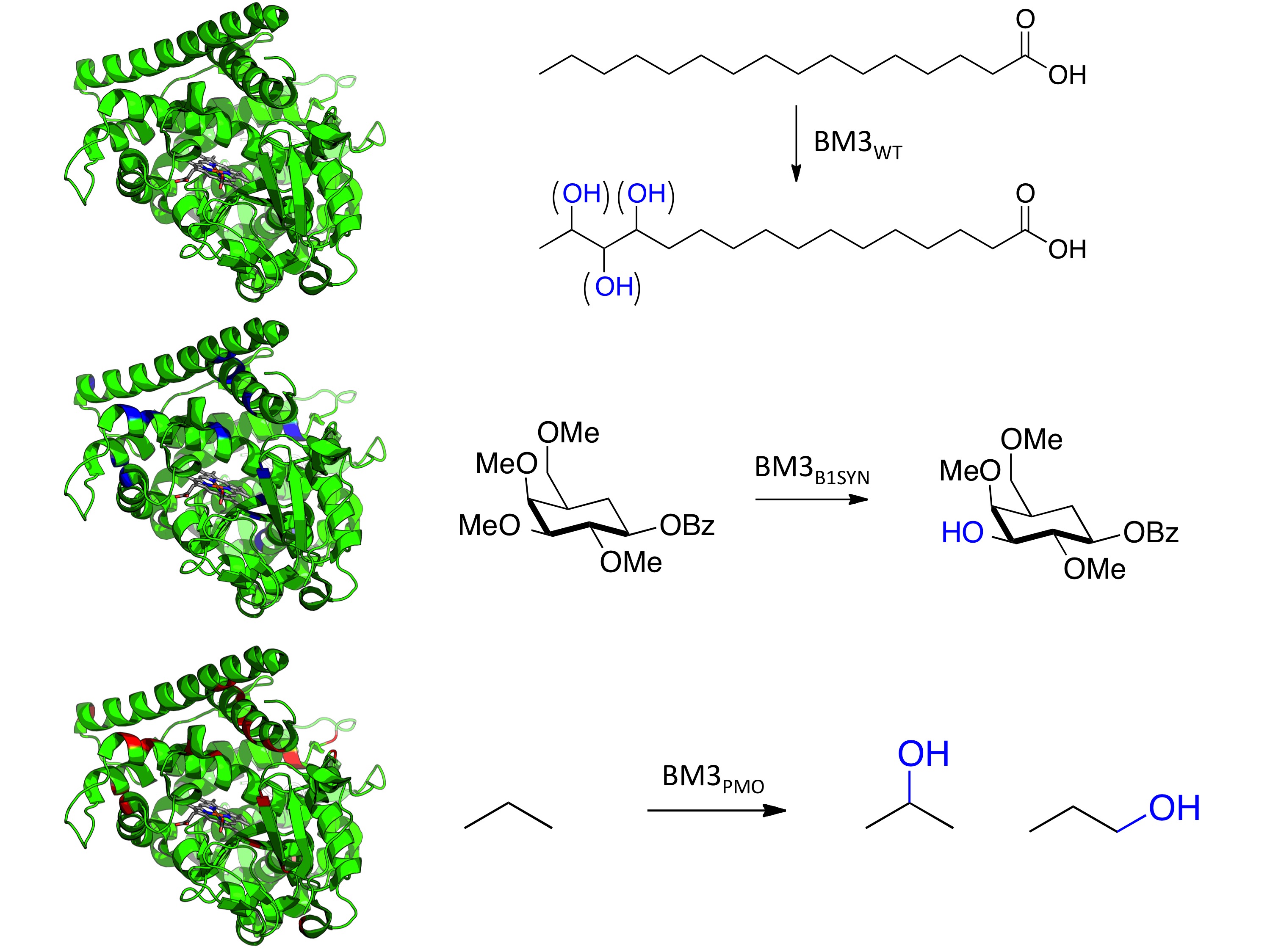
We have been making new P450s by directed evolution for a number of years. We have accumulated a remarkable library of catalysts with widely different substrate selectivities and patterns of reactivity. For example, BM3 variants that perform the following reaction types have been developed:
| • | hydroxylation of gaseous alkanes to yield short chain alcohols |
| • | installation of chiral hydroxyl groups in complex drug scaffolds |
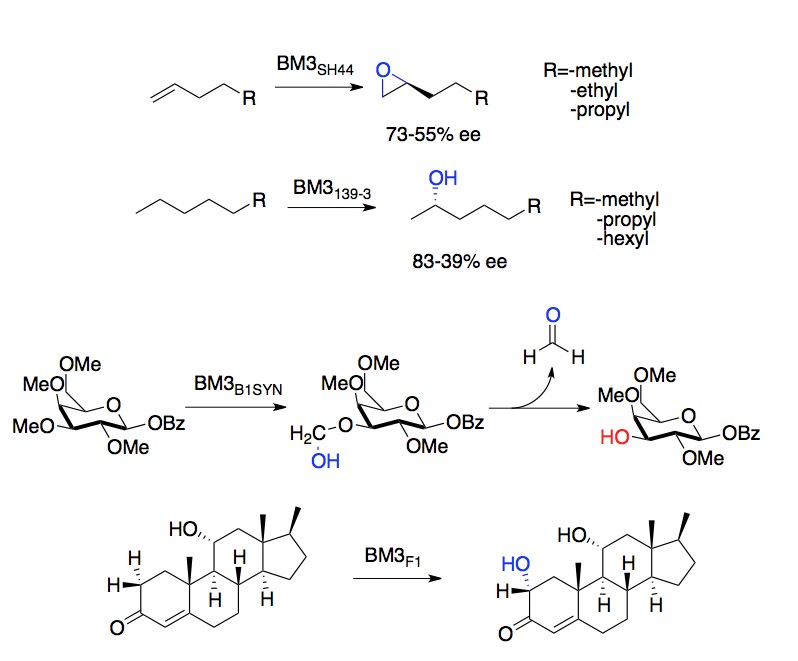
| • | selective deprotection of -OMe groups in globally protected sugars | • | stereoselective epoxidation of alkenes | • | mimicry of human P450s for production of drug metabolites |
Beyond the practical benefits of making useful catalysts, work on this unique model system has provided a number of fundamental insights into protein evolution and P450 enzymology. BM3 has served as a convenient system with which to observe protein evolution in the laboratory, which we think can tell us a lot about how proteins evolve and diversify in nature.
For example, mutations introduced via recombination between relatives tend to be much less destabilizing to protein folding than do random mutations. Moreover, stabilizing mutations are often additive in their effects—particularly in chimeric proteins. Even BM3 variants evolved to meet practical goals frequently allow us to ask more fundamental questions; studies of a series of variants evolved to hydroxylate small alkanes allowed us to observe the structural and catalytic changes that occur during the evolution of an initially ‘promiscuous’ activity.
Papers to get started:
"Evolutionary History of a Specialized P450 Propane Monooxygenase," R. Fasan, Y. T. Meharenna, C. D. Snow, T. L. Poulos, F. H. Arnold. Journal of Molecular Biology 383(5), 1069-1080 (2008).
This paper traces the structural and functional changes through a series of variants first evolved to catalyze hydroxylation of C-8 alkanes, which served as evolutionary starting points for variants capable of hydroxylating propane. The structural changes observed were at first quite modest, but became more significant as propane became the preferred substrate.
"Engineered Bacterial Mimics of Human Drug Metabolizing Enzyme CYP2C9," A. Rentmeister, T. R. Brown, C. D. Snow, M. N. Carbone, F. H. Arnold. ChemCatChem 3,1065-1071 (2011).
Human drug metabolites are of crucial medical importance, since metabolites are frequently active compounds in their own right, and may have toxic effects, however they are often produced in vanishing amounts. Here we show that BM3 can be used to produce preparative-scale quantities of metabolites that are identical to those produced by native human P450s.
"Chemoenzymatic Elaboration of Monosaccharides Using Engineered Cytochrome P450BM3 Demethylases," J. C. Lewis, S. Bastian, C. S. Bennett, Y. Fu, Y. Mitsuda, M. M. Chen, W. A. Greenberg, C.-H. Wong, F. H. Arnold. Proceedings of the National Academy of Sciences 106, 16550-16555 (2009).
One great challenge of carbohydrate synthesis is the development of methods for the selective deprotection of many chemically similar hydroxyl groups found in monosaccharides. Here we introduce a robust method to selectively deprotect methyl protecting groups in sugars, which can then be used in downstream applications.

