New apps for old enzymes: beyond hydroxylation
As noted in many recent reviews, more and more industrial processes are beginning to take advantage of enzymatic methods in the synthesis of drugs and fine chemicals. Directed evolution methods have been used to develop enzyme catalysts to install chiral functionalities in important drugs such as sitagliptin and bocepravir.
Laboratory-evolved cytochrome P450s will be useful for rapid customization of lead compounds in early-phase pharmaceutical development. Indeed, work in our lab has demonstrated that P450 enzymes can be used to install hydroxyl groups at essentially any desired position in aliphatic substrates. Consequently, these enzymes may open up whole new regions of 'chemical space' that could not be explored in previous medicinal chemistry efforts.
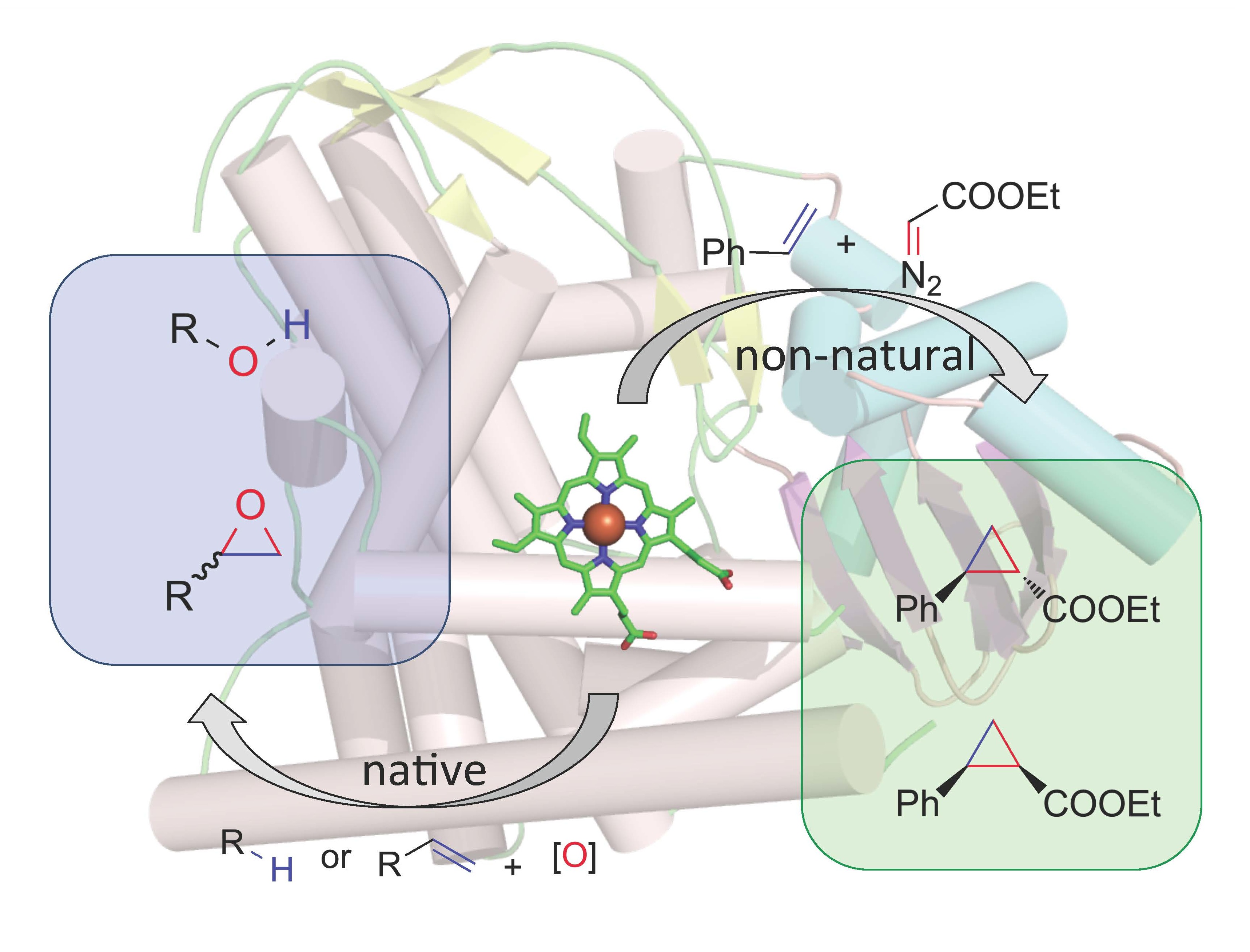
We are now using P450 enzymes to catalyze whole new reactions. We recently demonstrated this idea with a synthetically important C-C bonding forming reaction that has no biological counterpart. Reasoning that P450s might be able to form metal-carbenoids, we demonstrated that engineered cytochrome P450BM3 variants catalyze highly diastereo- and enantioselective cyclopropanation of styrenes from diazoester reagents. We are excited about adapting existing enzymes for catalysis of synthetically important reactions not previously observed in Nature.
Some of our recent papers are here:
"Olefin Cyclopropanation via Carbene Transfer Catalyzed by Engineered Cytochrome P450 Enzymes," P. S. Coelho, E. M. Brustad, A. Kannan, F. H. Arnold. Science xpress, December 20, 2012. doi: 10.1126/science1231434
A bacterial cytochrome P450 has been readily engineered to catalyze highly stereoselective carbene transfers to aryl-substituted olefins, enabling cyclopropanation through a route not observed in Nature.
"Optimizing Non-Natural Protein Function with Directed Evolution," E. M. Brustad, F. H. Arnold. Current Opinion in Chemical Biology 15, 201-210 (2011)
Here we take a look at some of the emerging technologies for diversifying protein function and discuss how directed evolution can help.
"Cytochrome P450: Taming a Wild Type Enzyme," S. T. Jung, R. Lauchli, F. H. Arnold. Current Opinion in Biotechnology 22, 809-817 (2011).
Progress in the engineering of cytochrome P450 enzymes, in particular BM3 is reviewed. We also pose the question, what makes proteins evolvable? And, why are P450s particularly amenable to directed evolution?
"Enzymatic Functionalization of Carbon-Hydrogen Bonds,"J. C. Lewis, P. S. Coelho, F. H. Arnold. Chemical Society Reviews 40,2003-2021 (2011).
Cytochrome P450 enzymes are placed in the context of Nature’s other oxidation catalysts. We describe the utility of each class and their applications in biotechnology.

