Engineering P450 BM3 for noninvasive imaging of neurotransmitters
Fei Sun, Jane Wang
Molecular probes that allow in vivo imaging of neural signaling events are important for investigating the basis of brain activity and neuron disorders. Taking advantage of the malleable nature of the substrate binding pocket of P450 BM3 and the power of directed evolution, we successfully developed P450-based magnetic resonance imaging (MRI) contrast agents for in vivo detection of neurotransmitters including dopamine and serotonin. These genetically-encodable biosensors potentially allow us to noninvasively visualize neural activity in living animals.
We will continue to engineer P450-based sensors with high specificity and sensitivity for molecules of interest, using powerful new capabilities for incorporating non-natural hemes and non-natural amino acids. We are also branching out into new protein engineering applications in neuroscience.
Papers to get started:
"Directed Evolution of a Magnetic Resonance Imaging Contrast Agent for Noninvasive Imaging of Dopamine," M. G. Shapiro, G. G. Westmeyer, P. A. Romero, J. O. Szablowski, B. Kuster, A. Shah, C. R. Otey, R. Langer, F. H. Arnold, A. Jasanoff. Nature Biotechnology 28, 264-270 (2010).
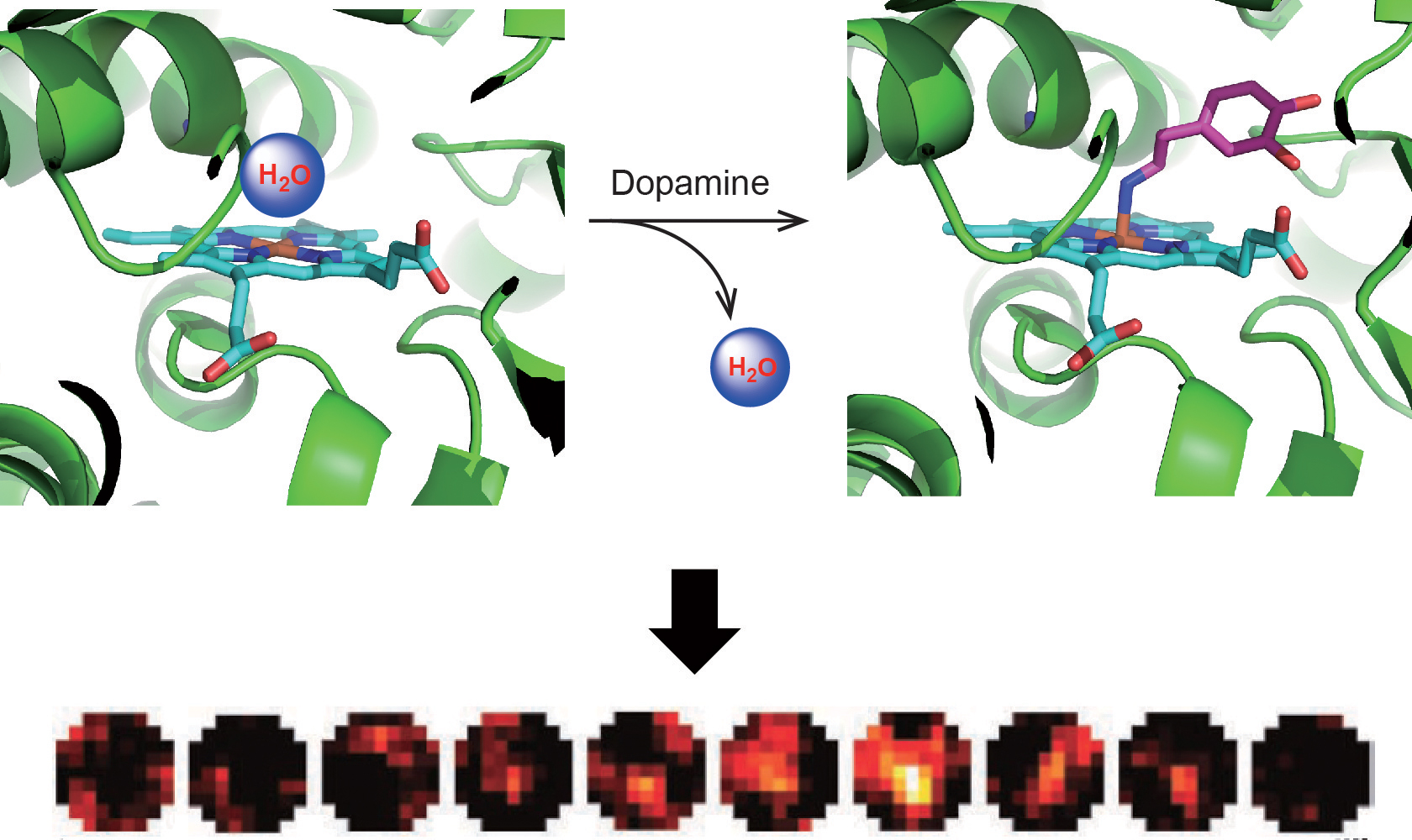
This paper describes the first genetically encodable fMRI sensor for neurotransmitter dopamine detection. The sensor was derived from the heme domain of P450-BM3 (BM3h). Through several rounds of directed evolution, the resulting P450-based MRI sensor is capable of imaging dopamine in brains of live animals with a good specificity. This work demonstrates the feasibility of reengineering P450 for broad applications on biomedical research.
"Metal-Substituted Protein MRI Contrast Agents Engineered for Enhanced Relaxivity and Ligand Sensitivity," V. S. Lelyveld, E. Brustad, F. H. Arnold, A. Jasanoff. Journal of the American Chemical Society 133, 649-651 (2010).
In order to augment the proton relaxivity of agents derived from BM3h, manganic BM3h was obtained by coexpressing the heme domain of P450 BM3 with the bacterial heme transporter ChuA in Escherichia coli and supplementing the growth medium with Mn. The Mn-substituted BM3h exhibits significantly increased relaxivity upon binding of arachidonic acid, a natural ligand for the P450 enzyme. This work demonstrates more generally that metal substitution techniques can be an important component of efforts to engineer effective protein-based sensors for molecular MRI.
"Structure-Guided Directed Evolution of Highly Selective P450-Based Magnetic Resonance Imaging Sensors for Dopamine and Serotonin," E. M. Brustad, V. S. Lelyveld, C. D. Snow, N. Crook, S. T. Jung, F. M. Martinez, T. J. Scholl, A. Jasanoff, F. H. Arnold. Journal of Molecular Biology 422, 245-262 (2012).
This paper describes powerful P450-based sensors for dopamine and serotonin. Several high resolution crystal structures of fMRI biosensors allowed us to see how ligand binding changed over the course of the directed evolution. These structures also help guide further engineering efforts.

